Proton therapy can deliver highly conformal dose distributions to a tumour target while minimizing dose to tissues outside the target volume. Creating treatment plans that realize this strength is a top priority for dosimetrists and medical physicists.
Protons deposit dose in a fundamentally different way to X-rays, another type of external-beam radiation therapy. As a proton reaches the end of its trajectory, the rate at which its energy is transferred to tissue – its linear energy transfer (LET), expressed in keV/µm – increases.
The relative biological effectiveness (RBE) captures the biological implications of increasing LET, and a fixed RBE value of 1.1 is often applied for clinical proton treatments. But proton RBE is dependent on many other factors, including clinical endpoints, tissue type, fractionation scheme, patient-specific radiosensitivity, physical dose, and uncertainties in experimental measurements. As a result, using a fixed RBE value in proton therapy likely underestimates RBE in high-LET locations, which could result in an increased risk of radiation-induced toxicities.
Still, LET is strongly correlated with RBE and is a key factor for determining variable RBE in proton therapy. As such, researchers are investigating approaches for calculating and evaluating LET during treatment planning. These biological treatment planning tools are limited, however, and until they are developed and studied further, clinics must identify their own treatment planning practices to minimize LET outside of target volumes, says Austin Faught, a medical physicist at St Jude Children’s Research Hospital in Tennessee.
“How to influence the [LET distribution] is an active area of research, and there are some great methods under development,” Faught explains. “The problem that we face is that those are not readily available without custom software developed in-house or through special research versions of vendor-provided applications … [and there are] few studies providing quantitative guidance on what we should aim for.”
Treatment planning strategies
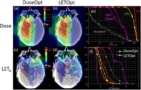
In a step toward LET-based plan evaluation and optimization for photon therapy, Faught and his team performed a survey of planning strategies that are commercially available to clinical teams for intensity-modulated proton therapy (IMPT). Their study, reported in the Journal of Applied Clinical Medical Physics, introduces some guidance for proton therapy treatment planners. “We wanted to look at some readily available treatment planning techniques and how they may affect LET,” Faught explains.
The researchers evaluated the differences in dose-weighted LET (LETd) between eight forward-based treatment planning approaches applied to a cylindrical water phantom and four paediatric brain tumour cases (Faught notes that radiation-induced toxicities are a focus area for the team). They compared these planning strategies to a plan using opposed lateral beams (for the phantom) or to the original clinical plan (for patients), using Monte Carlo secondary calculations to evaluate both the dose and LETd.
The researchers found that treatment field geometry was the biggest contributor to the location of high-LET areas. To mitigate the potential impact of biologic uncertainties associated with high LETd, they suggest that treatment planners use large intersection angles between treatment beams and avoid beams that stop immediately proximal to critical structures.
“This is great news as it means careful selection of the number of treatment fields and their orientation with respect to nearby health tissues can be effective,” Faught says. “With some conscious, upfront thought, that’s something all treatment planners can take into consideration during the planning process.”
The researchers also found that using a range shifter significantly reduced the mean LETd in the clinical target volume. As a result, they recommend using range shifters and alternative strategies of spot-placement restrictions sparingly, and only when clinics can calculate the resulting LETd to evaluate against alternate planning strategies.
Because of the study’s small sample size, the researchers couldn’t establish a clear trend in LETd variations in the clinical cases. They did not evaluate the relationship between changes in LET and a change in the probability of tumour control or normal tissue complications.
LET-based plans optimize proton therapy
While the effects of each planning approach on high-LET regions were modest, Faught says it’s important to recognize that the team’s treatment planning strategies and recommendations are evidence-based and can readily be worked into clinical practice.
“I hope that one of the takeaways is that we, as a field, would benefit from commercial tools that allow for the calculation of LET within the treatment planning system. Even better, we would love to have ways to optimize with LET in mind. This study was a good bridge until those tools are more widely available,” Faught says.













