A team of UK researchers has designed a novel endoscope that uses sound and light to image tissue samples on molecular scales, based around a detector that’s small enough to fit inside a medical needle. In their study, Wenfeng Xia and colleagues at King’s College London and University College London improved several key aspects of the photoacoustic imaging technique – ensuring fast imaging times without sacrificing the size of the equipment required.
Photoacoustic endoscopy is a cutting-edge technique that combines ultrasound with optical endoscopic imaging to build up 3D medical images. It works by sending out laser pulses through an endoscope’s optical fibre, which are absorbed by microscopic structures inside the body. As they absorb the light’s energy, these structures generate acoustic waves – which are themselves picked up by a piezoelectric ultrasound detector and converted into images.
The technique allows researchers to pick out a wide range of microscopic structures: from individual cells to strands of DNA. It already addresses many limitations of purely optical endoscopes, including their inability to penetrate through more than a few layers of cells. Yet despite these advantages, photoacoustic endoscopy still faces a trade-off: in order to achieve higher imaging speeds, it requires bulkier, more costly ultrasound detectors, limiting its applicability in minimally-invasive surgery.
To address this challenge, Xia’s team has introduced a new approach. The design – reported in Biomedical Optics Express – first features a “digital micromirror” containing an array of nearly one million microscopic mirrors, whose positions can each be rapidly adjusted. The researchers used this setup to precisely shape the wavefronts of laser beams used to scan over samples.
Instead of a piezoelectric ultrasound detector, the researchers introduced a far less bulky optical microresonator. Fitting onto the tip of an optical fibre, this device contains a deformable epoxy spacer sandwiched between a pair of specialized mirrors. The incoming ultrasound waves deform the epoxy, altering the distance between the mirrors. This leads to changes in the microresonator’s reflectivity changes as the endoscope is raster-scanned over samples.
When interrogated with a second laser, delivered to the tip of the endoscope along a separate optical fibre, these variations alter the amount of light reflected back along the fibre. By monitoring these changes, an algorithm developed by the team can build up images of the sample and use them to calculate how the scanning laser’s wavefront can be adjusted to produce more optimal images. With this information, the digital micromirror is adjusted accordingly, and the process repeats.
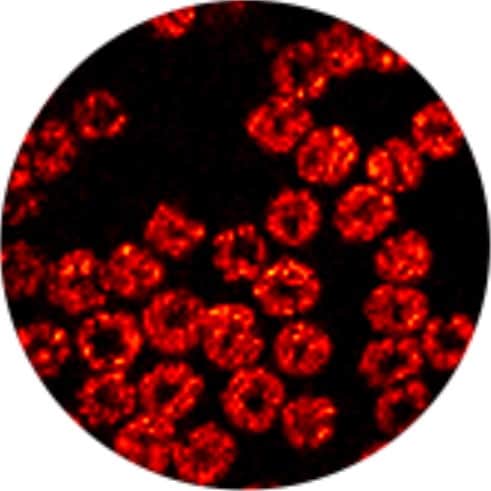
By adjusting the focal length of the scanning laser beam, the endoscope can also scan samples from their surfaces down to depths of 20 µm – allowing Xia’s team to build up optimized 3D images in real time.
To demonstrate these unique capabilities, the researchers used their device to image a cluster of mouse red blood cells, spread across an area roughly 100 µm across. By stitching together a mosaic of photoacoustic scans, the endoscope produced 3D images of the cells, at speeds of around 3 frames per second.
Based on their success, Xia and colleagues now hope that their endoscope could inspire new advances in minimally-invasive surgery – allowing clinicians to assess the molecular and cellular-scale makeup of tissues in real time. In future studies, the team will aim to explore how artificial intelligence could help to enhance photoacoustic imaging speeds even further.













