Blocked blood vessels caused by cardiovascular disease can lead to serious outcomes including heart attack or stroke. The condition can be treated by surgically bypassing the blockage using a vessel from elsewhere in the patient’s body. When this is not feasible, a synthetic vascular graft is generally used. Synthetic grafts have high rates of failure, however, due to chronic inflammation caused by the body rejecting a foreign substance. Another option is human tissue-engineered vascular grafts (TEVGs), which show promising in vivo results, but require lengthy, complex and expensive processes to create.
Now, researchers at INSERM’s Lab for the Bioengineering of Tissues (BioTis U1026) at the University of Bordeaux have successfully fabricated small-diameter TEVGs using human amniotic membrane (HAM) threads combined with a textile-inspired weaving strategy. Describing the process in Biofabrication, they claim that these grafts have remarkable properties that justify moving into in vivo laboratory animal testing.
HAM, the innermost layer of membranes surrounding a foetus during development, provides a viable biological “scaffold” for tissue engineering. It exhibits anti-inflammatory properties, anti-microbial effects, low immunogenicity (the ability to provoke an immune response), blood compatibility, suture-holding capacity and high mechanical strength. It also routinely discarded by hospitals and, consequently, is widely available and affordable.
Yarn production
Principal investigator Nicolas L’Heureux and colleagues created HAM yarns from foetal membranes collected from consenting patients following caesarean deliveries. They prepared the membranes for use by rinsing the tissues repeatedly in distilled water, cutting the membranes into 10 x 18 cm rectangular sheets, and manually separated the amnion and chorion (inner and outer membranes). A motorized cutting device then sliced the HAM sheets into 5- or 10-mm-wide ribbons.
To create mechanically strong threads, the researchers attached these ribbons to a rotating device that twisted them at 5, 7.5 or 10 revolutions/cm. The yarn diameter decreased after twisting, plateauing at 7.5 revolutions/cm, while the ultimate tensile stress increased significantly after twisting at 7.5 and 10 revolutions/cm.
The HAM yarns (ribbons and threads) were dried at room temperature, spooled and stored at -80°C, a process known as devitalization as it kills the cells. When needed, the researchers rehydrated the yarns in distilled water.
Since their aim was to provide an off-the-shelf implant, the researchers examined the effects of decellularization and sterilization with gamma irradiation on the HAM ribbons. Histology showed that decellularization effectively removed cellular components that remained after devitalization, did not affect the HAM strength and increased its stretchability.
When dry HAM ribbons were gamma-sterilized, they became thinner, stiffer and less stretchable. Keeping the HAM ribbons hydrated during sterilization prevented many of these effects. The researchers observed that wet sterilization did not impact the ability of HAM to support endothelial cell attachment and growth.
Weaving the vessels
In the final step, the researchers assembled the HAM yarns into TEVGs. They used a custom-made circular loom to weave TVEGs around a stainless steel mandrel. To create a woven tube, a circumferential yarn (the “weft”) was inserted between a movable and a fixed set of tensioned longitudinal ribbons (the “warp”). The two warp sets were moved to cross over the weft, the circumferential yarn was run again between them, and the process was repeated 50 times.
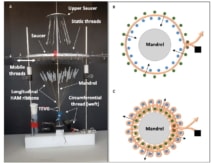
The team used 51 longitudinal ribbons (5 mm wide) and one double-ribbon circumferential thread to weave TVEGs with an average internal diameter of 4.4 ± 0.2 mm. The woven TEVGs were mechanically robust, with superior suture retention strength and average burst pressure to those of human internal mammary arteries, the preferred vessel for heart bypass surgery.
However, because the transmural permeability was potentially too high, the team produced a second set of TVEGs using 10-mm-wide longitudinal ribbons and the same circumferential thread design. This created TEVGs with a larger internal diameter of 5.2 ± 0.4 mm. The walls displayed increased yarn density and drastically reduced transmural permeability. The burst pressure increased and suture retention strength remained the same.
“Combining inexpensive HAM with a weaving assembly method decreases the costs to produce TEVGs by avoiding the use of cells and bioreactors, which are necessary in other methods,” write the authors. “No assembly method used today allows inexpensive production of HAM-based TVEGs with proven mechanical properties compatible with arterial implantation.”

Towards in vitro blood vessel fabrication
The researchers point out that textile-inspired assembly strategies using weaving, knitting and braiding are already widely used to produce medical devices. Thus it should not be difficult to design machines to handle HAM yarn and enable mass production of TVEGs after successful clinical studies are performed. They add that yarn diameter, mechanical strength and other mechanical properties can be easily modified to meet various specification requirements.
Next, the researchers plan to assess the impact of decellularization and post-assembly gamma sterilization of the various properties of the woven TVEG, particularly with respect to permeability and stretchability.