Neurodegenerative diseases, characterized by a progressive loss of brain function, result primarily from synaptic loss and neuronal cell death in the central nervous system. อัลไซเม disease (AD) is arguably the most common neurodegenerative disorder leading to dementia.
The shortcomings of treatments reflect that scientists have never fully understood how Alzheimer’s develops. Scientists also don’t know why women account for nearly two-thirds of cases.
นักวิจัยที่ เอ็มไอที และ การวิจัย Scripps have discovered a hint to the molecular aetiology of Alzheimer’s disease; this clue may also explain why women are more susceptible to the disease.
Researchers discovered a particularly harmful, chemically modified form of an inflammatory immune protein called complement C3 was present at much higher levels in the brains of women who had died with the disease compared to men who had died with the disease. They also showed that ฮอร์โมนหญิง—which drops in production during วัยหมดประจำเดือน—typically protects against the creation of this form of complement C3.
Study senior author Stuart Lipton, MD, Ph.D., professor and Step Family Foundation Endowed Chair in the Department of Molecular Medicine at Scripps Research and a clinical neurologist in La Jolla, California, said, “การค้นพบใหม่ของเราชี้ให้เห็นว่าการปรับเปลี่ยนทางเคมีของส่วนประกอบของระบบเสริมช่วยขับเคลื่อนโรคอัลไซเมอร์ และอาจอธิบายได้อย่างน้อยในบางส่วนว่าทำไมโรคนี้จึงส่งผลกระทบส่วนใหญ่กับผู้หญิง”
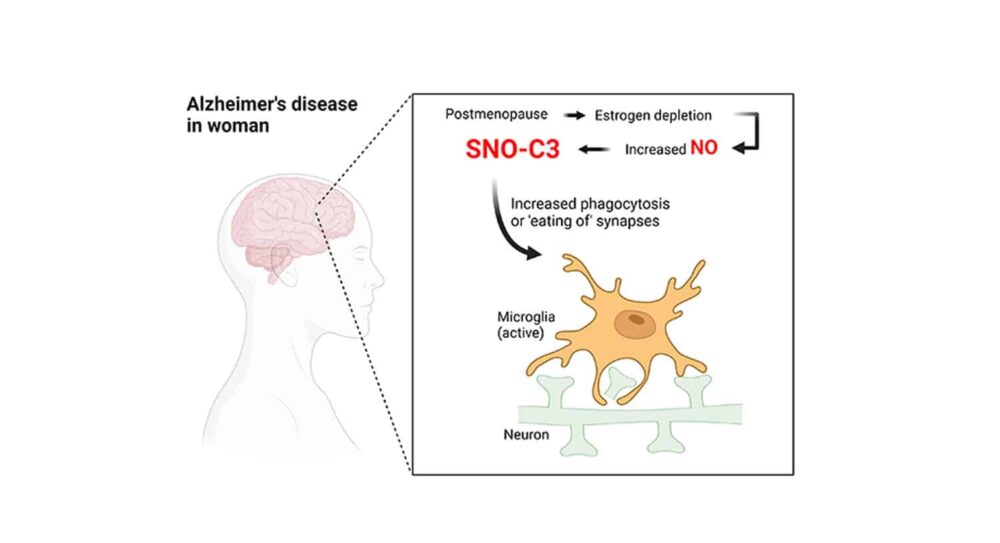
ห้องปฏิบัติการของลิปตันจะตรวจสอบกระบวนการทางชีวเคมีและโมเลกุล เช่น โปรตีน S-ไนโตรซิเลชัน ซึ่งส่งผลให้เกิดเวอร์ชันเสริมของ C3 ที่ได้รับการดัดแปลง ซึ่งอาจเป็นสาเหตุของ ความผิดปกติของระบบประสาท. This chemical process, which produces a modified “SNO-protein” when a nitric oxide (NO)-related molecule attaches securely to a sulfur atom (S) on a specific amino acid building block of proteins, was first found by Lipton and his coworkers.
Small clusters of atoms, like NO, frequently modify proteins in cells. These alterations typically activate or inactivate a target protein’s functions. Because of technical difficulties, S-nitrosylation has received less attention than other protein modifications. Still, Lipton hypothesizes that the “SNO-storms” of these proteins may play a significant role in developing Alzheimer’s disease and other neurodegenerative diseases.
Lipton’s lab investigates biochemical and molecular processes, such as protein S-nitrosylation, which result in a modified version of complement C3, which may be the cause of neurodegenerative disorders. This chemical process, which produces a modified “SNO-protein” when a nitric oxide (NO)-related molecule attaches securely to a sulphur atom (S) on a specific amino acid building block of proteins, was first found by Lipton and his coworkers.
Small clusters of atoms, like NO, frequently modify proteins in cells. These alterations typically activate or inactivate a target protein’s functions. Because of technical difficulties, S-nitrosylation has received less attention than other protein modifications. Still, Lipton hypothesizes that the “SNO-storms” of these proteins may play a significant role in developing Alzheimer’s disease and other neurodegenerative diseases.
In the latest study, the scientists measured the amount of proteins changed in 40 postmortem human brains using brand-new techniques for identifying S-nitrosylation. The brains were divided equally between males and females, with half coming from persons who had died of Alzheimer’s disease and the other half coming from people who hadn’t.
The researchers discovered 1,449 distinct proteins that had been S-nitrosylated in these brains. Numerous proteins that have already been connected to Alzheimer’s disease were among those that were most often altered, including complement C3. Surprisingly, female Alzheimer’s brains had S-nitrosylated C3 (SNO-C3) levels that were more than six times higher than those of male Alzheimer’s brains.
The complement system is an evolutionarily older part of the human ระบบภูมิคุ้มกัน. It consists of a family of proteins, including C3, that can activate one another to drive inflammation in the “complement cascade.”
For more than 30 years, researchers have known that, when compared to neurologically healthy brains, Alzheimer’s brains exhibit higher amounts of complement proteins and other inflammatory indicators. More recent studies have demonstrated, in particular, how complement proteins can cause brain-resident immune cells known as microglia to degrade synapses. At these junctions, neurons communicate with one another.
Many researchers now suspect this synapse-destroying mechanism partly underlies Alzheimer’s disease. Loss of synapses has been demonstrated to be a significant correlate of cognitive decline in Alzheimer’s brains.
Why would female brains with Alzheimer’s have a higher prevalence of SNO-C3? The researchers proposed the hypothesis that oestrogen protects women’s brains from C3 S-nitrosylation—and that this protection is lost when oestrogen levels sharply decline with menopause. There has long been evidence that the female hormone estrogen can have brain-protective effects under some circumstances. This theory was validated by experiments using cultured human เซลล์สมอง, which showed that SNO-C3 increases as oestrogen (-estradiol) levels fall due to activating an enzyme that produces NO in brain cells. Synapses are destroyed by microglia when SNO-C3 levels rise.
ลิปตัน พูดว่า, “เหตุใดผู้หญิงจึงมีแนวโน้มที่จะเป็นโรคอัลไซเมอร์จึงเป็นปริศนามานานแล้ว แต่ฉันคิดว่าผลลัพธ์ของเราเป็นส่วนสำคัญของปริศนาที่อธิบายอย่างมีกลไกถึงความอ่อนแอที่เพิ่มขึ้นของผู้หญิงเมื่ออายุมากขึ้น”
การอ้างอิงวารสาร:
- Hongmei Yang, Chang-ki Oh, et al. Mechanistic insight into female predominance in Alzheimer’s disease based on aberrant protein S-nitrosylation of C3. วิทยาศาสตร์ก้าวหน้า. ดอย: 10.1126/sciadv.ade0764