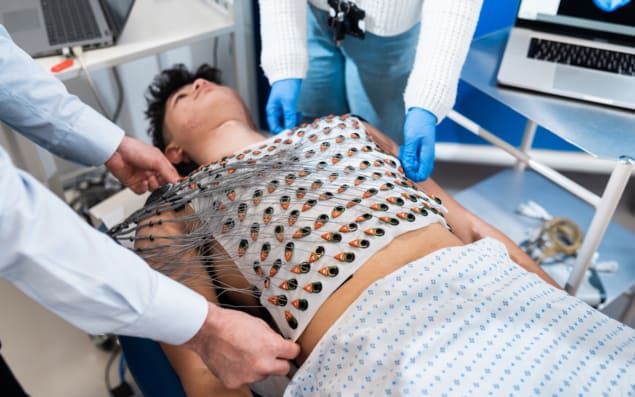
A reusable vest that generates high-resolution maps of the heart’s electrical activity could help identify people at risk of sudden cardiac death. Developed by a team headed up at University College London (UCL), the vest combines electrical data recorded by its 256 sensors with detailed MR images of heart structures to create real-time maps of cardiac activation and recovery patterns.
There are 4–5 million cases of sudden cardiac death each year worldwide, the majority caused by heart rhythm disorders. Implantable cardioverter defibrillators that monitor the heart’s rhythm and, if needed, shock it back into a normal rhythm can save lives. But an implanted device comes with its own risks, making it essential to identify how a particular cardiac structural abnormality can affect the risk of sudden cardiac death.
While detailed electrophysiological mapping can quantify this risk, such procedures are time consuming, costly and often highly invasive. Instead, the researchers propose the use of electrocardiographic imaging (ECGI) – a non-invasive technique that combines cardiac and torso geometry with body surface potentials recorded from multiple electrodes. As ECGI is high resolution and corrects for anatomy, it can detect information-rich electrical phenomena that would be missed by conventional 12-lead ECG.
“The ECG only collects signals from 12 limited points on the heart’s surface – that is not enough to generate a 3D map of all the flow of electrical data across the heart,” explains the vest’s developer Gabriella Captur. “To construct such a map you need a dense and high-resolution data collection method like ECGI. With ECGI we have 256 leads across the front and back and we process these to yield 1000 individual nodes over each heart.”
“12-lead ECG is like looking at the night sky with the naked eye,” Captur tells Physics World. “The ECGI vest is like looking into deep space using the James Webb telescope when suddenly the whole universe is teeming with stars.”
Unlike previous ECGI approaches that used CT for anatomical imaging, the new vest uses radiation-free cardiovascular magnetic resonance (CMR) to provide data on cardiac structure and function.
“MRI is the ‘Rolls Royce’ of cardiac imaging. It tells us which, if any, sections of the heart muscle wall are dead, scarred, inflamed, weakened or injured,” says Captur. “For the first time we can say precisely how these changes in the heart muscle wall are impacting the electrics of the heart, with obvious advantages in terms of predicting likelihood of dangerous heart rhythms or response to therapy.”
Test the vest
The ECGI vest, described in the Journal of Cardiovascular Magnetic Resonance, is a cotton garment embroidered with 256 textile-based dry electrodes (2 × 2 cm), with a graphite-snap connector in each electrode to connect the ECG lead. As it uses dry electrodes, rather than metallic electrodes that require a gel layer next to the skin, the vest (minus the ECG leads) is fully washable and reusable – providing a cost-effective screening tool.
For ECG data collection, the electrode vest is secured around the patient’s chest, with an inflatable gilet worn over the top to maximize skin–electrode contact. Body surface potentials are recorded for 5 min, after which the electrode vest is swapped for a “mirror vest” for CMR scanning. This mirror vest, in which each electrode is replaced by a CMR-safe fiducial marker, avoids the need to disconnect all 256 ECG leads after each recording and thus streamlines the process. The CMR scan is then performed using a 3T or 1.5T MRI system.
The researchers tested the same reusable vest on 77 participants, including 27 young healthy volunteers and 50 older persons. All ECGI recordings were completed without complications and took less than 10 min per participant.

After data collection, the team reconstructed epicardial electrograms and used these to compute local electrophysiological parameters, including cardiac activation time, repolarization time and activation recovery intervals. The total post-processing – including segmentation of heart–torso geometries from the CMR scan, signal averaging and reconstruction of epicardial maps – took approximately 15 min per participant.
The researchers performed variability studies on 20 participants, which included repeating all steps in the post-processing pipeline. The CMR-ECGI workflow showed excellent reproducibility, with low intra- and inter-observer variability in measured ECGI parameters. The team also examined the scan/rescan variability in eight participants, by repeating the ECGI recording and CMR scan at least three months after the original measurements, observing high repeatability.
The vest measurements revealed differences between young and older participants, with electrophysiological parameters such as repolarization time and activation recovery interval prolonged in the older compared to the younger group. The team suggest that this may be due to age-related changes in cardiac ion channels and calcium handling that would alter the action potential duration and recovery.

Wearable ECG provides continuous cardiac monitoring
The ECGI vest has now been used in 800 patients, and the team is currently using it in people with heart muscle disorders. “We are using this vest to study the hearts of patients with hypertrophic cardiomyopathy (thickened heart muscle) to understand if the ECGI signature can identify those carrying the gene mutation before the thickening starts and to see whether the ECGI signature can predict the risk of sudden death,” says Captur.
“We are also using the vest at rest and during exercise to study the hearts of patients with weak hearts (dilated cardiomyopathy), to understand whether scar in a specific section of the heart muscle wall increases one’s risk of having a cardiac arrest.”
Captur has patented the vest in the US and is working with g.tec medical engineering, which created the prototype and is now manufacturing the vest for other research centres to purchase and use.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://physicsworld.com/a/cardiac-vest-creates-detailed-map-of-the-hearts-electrical-activity/
- :has
- :is
- :not
- $UP
- 1
- 12
- 20
- 27
- 3d
- 50
- 77
- a
- abnormality
- AC
- across
- Action
- Activation
- activity
- advantages
- affect
- After
- All
- also
- an
- anatomy
- and
- any
- approaches
- approximately
- ARE
- around
- arrest
- AS
- At
- averaging
- back
- BE
- been
- before
- between
- body
- but
- by
- Calcium
- CAN
- carrying
- cases
- caused
- centres
- Changes
- channels
- click
- collection
- College
- combines
- comes
- compared
- Completed
- Compute
- Connect
- construct
- consuming
- contact
- continuous
- conventional
- cost-effective
- costly
- could
- create
- created
- creates
- Currently
- Dangerous
- data
- dead
- Death
- deep
- described
- detailed
- detect
- developed
- Developer
- Development
- device
- differences
- disorders
- dry
- due
- duration
- during
- each
- eight
- enough
- essential
- excellent
- Exercise
- eye
- First
- first time
- flow
- For
- from
- front
- fully
- function
- generate
- generates
- Group
- Handling
- Have
- having
- headed
- healthy
- Heart
- help
- High
- high-resolution
- highly
- How
- HTTPS
- identify
- if
- image
- images
- Imaging
- impacting
- in
- included
- Including
- Increases
- individual
- information
- instead
- Institute
- into
- invasive
- involved
- issue
- IT
- ITS
- james
- jpg
- layer
- lead
- Leads
- least
- less
- like
- likelihood
- Limited
- Lives
- local
- London
- looking
- Low
- Majority
- Making
- manufacturing
- map
- mapping
- Maps
- marker
- max-width
- Maximize
- May..
- measurements
- medical
- method
- million
- min
- mirror
- missed
- Monitor
- months
- mr
- MRI
- multiple
- muscle
- Need
- needed
- New
- next
- night
- nodes
- normal
- now
- obvious
- of
- often
- older
- on
- only
- open
- or
- original
- Other
- over
- own
- parameters
- participant
- participants
- particular
- patented
- patients
- patterns
- People
- per
- performed
- persons
- Peter
- Physics
- Physics World
- pipeline
- plato
- Plato Data Intelligence
- PlatoData
- points
- potential
- potentials
- precisely
- predict
- predicting
- previous
- procedures
- process
- propose
- prototype
- provide
- provides
- providing
- purchase
- rather
- real-time
- recorded
- recording
- recovery
- replaced
- require
- research
- researchers
- Resolution
- resonance
- response
- REST
- reusable
- Revealed
- Risk
- risks
- same
- Save
- say
- says
- scan
- scanning
- screening
- Section
- sections
- Secured
- see
- segmentation
- sensors
- showed
- Signal
- signals
- signature
- Skin
- Sky
- Space
- specific
- Staff
- Stars
- starts
- Steps
- streamlines
- structural
- structure
- structures
- Student
- studies
- Study
- such
- sudden
- suggest
- Surface
- system
- team
- technique
- telescope
- tells
- terms
- tested
- than
- that
- The
- then
- therapy
- These
- this
- those
- thumbnail
- Thus
- time
- to
- took
- tool
- top
- Total
- true
- UCL
- understand
- Universe
- university
- us
- use
- used
- uses
- using
- volunteers
- Wall
- washable
- we
- weak
- were
- when
- whether
- which
- whole
- with
- without
- workflow
- working
- world
- worldwide
- would
- year
- Yield
- You
- young
- Younger
- zephyrnet













