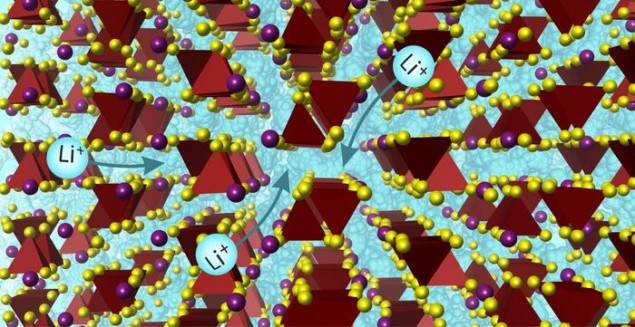
Researchers at the University of Liverpool, UK have developed a new solid-state battery electrolyte that conducts lithium ions so rapidly, it could compete with the liquid electrolytes found in today’s ubiquitous lithium-ion batteries. This high lithium-ion conductivity is a prerequisite for rechargeable energy storage, but it is unusual in solids, which are otherwise attractive for batteries because they are safer and quicker to charge.
The new electrolyte has the chemical formula Li7Si2S7I and contains ordered sulphide and iodide ions arranged in both a hexagonal and cubic-close-packed structure. This structure makes the material highly conductive because it facilitates the movement of lithium ions in all three dimensions. “One could envisage it as a structure that allows lithium ions to have more ‘options’ to choose from for movement, which means they are less likely to get stuck,” explains Matt Rosseinsky, the Liverpool chemist who led the research.
The right material with the right properties
To identify a material that facilitates this freedom of movement, Rosseinsky and colleagues used a combination of artificial intelligence (AI) and crystal structure prediction tools. “Our original idea was to create a new structural family of ion conductors inspired by the complex and diverse crystal structures of intermetallic materials, such as NiZr, in order to generate a wide range of potential sites for the lithium ions to move between,” Rosseinsky explains. AI and other software tools helped the team know where to look, though “the final decisions were always made by the researchers and not the software”.
After synthesizing the material in their laboratory, the researchers determined its structure with diffraction techniques and its lithium-ion conductivity with NMR and electrical transport measurements. They then demonstrated the lithium-ion conductivity efficiency experimentally by integrating the material into a battery cell.
Exploring unchartered chemistry
Rosseinsky’s research focuses on designing and discovering materials to support a transition to more sustainable forms of energy. This type of research involves a wide variety of techniques, including digital and automated methods, exploratory synthesis of materials with new structures and bonding, and the targeted synthesis of materials with real-world applications. “Our study brought all these directions together,” he says.
Discovering materials that differ from known ones is difficult, Rosseinsky adds, not least because any candidate materials must be experimentally realized in the lab. Once he and his colleagues have determined a material’s synthetic chemistry, they must then measure its electronic and structural properties. This inevitably requires interdisciplinary research: in the present work, Rosseinsky teamed up with colleagues in the Materials Innovation Factory, the Leverhulme Research Centre for Functional Materials Design, the Stephenson Institute for Renewable Energy and the Albert Crewe Centre and School of Engineering as well as his own department of chemistry.
Paddlewheel-like molecules push sodium ions through next-generation battery
Applicable to the larger field of battery research
The process the team developed, which is detailed in Science, could be applicable throughout the field of battery research and beyond, Rosseinsky says. “The knowledge gained in our work about how to favour fast ion motion in solids is relevant for materials other than those employed in lithium-ion batteries and is generalizable to other techniques that rely on ion-conducting materials,” he tells Physics World. “This includes proton or oxide ion conducting materials and solid-state fuel cells or electrolysers for hydrogen generation, as well as sodium and magnesium-conducing materials in alternative battery structures.”
The researchers say that Li7Si2S7I is likely just the first of many new materials accessible with their new approach. “There is thus much to do in defining which materials can be studied and how their ion transport properties connect to their structures and compositions,” Rosseinsky concludes.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://physicsworld.com/a/solid-state-battery-electrolyte-makes-a-fast-lithium-ion-conductor/
- :has
- :is
- :not
- :where
- $UP
- 160
- a
- About
- AC
- accessible
- Act
- Adds
- AI
- All
- allows
- alternative
- always
- and
- any
- applicable
- applications
- approach
- ARE
- arranged
- artificial
- artificial intelligence
- Artificial intelligence (AI)
- AS
- At
- Automated
- batteries
- battery
- BE
- because
- between
- Beyond
- Blue
- both
- brought
- but
- by
- CAN
- candidate
- cell
- Cells
- centre
- chemical
- chemistry
- Choose
- colleagues
- combination
- compete
- complex
- concludes
- conducting
- conducts
- Connect
- contains
- could
- create
- Crystal
- decisions
- defining
- demonstrated
- designing
- detailed
- determined
- developed
- differ
- difficult
- digital
- dimensions
- discovering
- diverse
- do
- efficiency
- electrolyte
- electrolytes
- Electronic
- employed
- energy
- Explains
- facilitates
- family
- FAST
- field
- final
- First
- focuses
- For
- forms
- formula
- found
- Freedom
- from
- Fuel
- fuel cells
- functional
- gained
- generate
- generation
- get
- Have
- he
- helped
- High
- highly
- his
- How
- How To
- http
- HTTPS
- hydrogen
- idea
- identify
- image
- in
- includes
- Including
- including digital
- inevitably
- information
- Innovation
- inspired
- Institute
- Integrating
- Intelligence
- into
- involves
- issue
- IT
- ITS
- jpg
- just
- Know
- knowledge
- known
- lab
- laboratory
- larger
- least
- Led
- less
- like
- likely
- Liquid
- Look
- made
- MAKES
- many
- material
- materials
- max-width
- means
- measure
- measurements
- methods
- more
- motion
- move
- movement
- moving
- much
- must
- New
- next-generation
- of
- on
- once
- ones
- or
- order
- original
- Other
- otherwise
- our
- own
- Physics
- Physics World
- plato
- Plato Data Intelligence
- PlatoData
- potential
- prediction
- prerequisite
- present
- process
- properties
- Push
- quicker
- range
- rapidly
- real world
- realized
- relevant
- rely
- Renewable
- represents
- requires
- research
- researchers
- right
- safer
- say
- says
- School
- showing
- Sites
- So
- sodium
- Software
- storage
- structural
- structure
- structures
- studied
- Study
- such
- support
- sustainable
- synthesis
- synthetic
- targeted
- team
- teamed
- techniques
- tells
- than
- that
- The
- their
- then
- These
- they
- this
- those
- though?
- three
- Through
- throughout
- thumbnail
- Thus
- to
- today’s
- together
- tools
- transition
- transport
- Transport properties
- true
- type
- ubiquitous
- Uk
- university
- used
- variety
- was
- WELL
- were
- which
- WHO
- wide
- Wide range
- with
- Work
- world
- zephyrnet