Neuroimaging has increased our understanding of brain function. Such techniques often involve measuring blood flow variations to detect brain activation, exploiting the fundamental interaction between the brain’s vascular and neuronal activities. Any alterations in this so-called neurovascular coupling are strongly linked to cerebral dysfunction. The ability to image cerebral microcirculation is particularly important, as neurodegenerative diseases such as dementia and Alzheimer’s involve dysfunction of the small cerebral vessels.
Researchers at Institute Physics For Medicine Paris (Inserm/ESPCI PSL University/CNRS) have now developed a method called functional ultrasound localization microscopy (fULM) that can capture cerebral activity at the micron scale. The team published the first micron-scale, whole-brain images of rodent vascular activity in Nature Methods, along with a detailed explanation of the fULM image acquisition and analysis procedures.
Unlike invasive electrophysiological or optical approaches to study brain function at the microscopic scale, ultrasound localization microscopy (ULM) can be non-invasive. The imaging technology tracks biocompatible micron-sized microbubbles injected into the blood circulation and by accumulating the tracks of millions of microbubbles, reconstructed images can reveal subtle changes in the cerebral blood volume with micron-sized accuracy, across large fields-of-view.
Researchers have previously used ULM to reveal microvascular anatomy at the whole-brain scale in rodents and humans. The spatial resolution of ULM is 16-fold better than that achieved with functional ultrasound imaging. But because the acquisition process is slow, ULM can only produce static maps of blood flow induced by the neuronal activity.
The fULM technique overcomes this limitation. In addition to imaging the brain microvasculature, the technique detects local brain activation by calculating the number and speed of microbubbles passing in each vessel. When a brain region activates, neurovascular coupling causes the blood volume to increase locally, dilating the vessels and allowing more microbubbles to pass. fULM provides local estimates of multiple parameters that characterize such vascular dynamics, including microbubble flow, speed and vessel diameters.
According to principal investigator Mickael Tanter and colleagues, integrating fULM into a cost-efficient, easy-to-use ultrasound scanner provides “a quantitative look at the cerebral microcirculatory network and its haemodynamic changes by combining a brain-wide spatial extent with a microscopic resolution and a 1 s temporal resolution compatible with neurofunctional imaging”.
In vivo studies
To demonstrate the fULM concept, the researchers first imaged laboratory rats with functional ultrasound (without contrast), followed by ULM in the same imaging plane. They combined sensory stimulations (whiskers deflections or visual stimulation) in anesthetized rats with continuous microbubble injection. For ULM, the rats received a continuous slow injection of microbubbles during a 20 min imaging session, leading to roughly 30 microbubbles per ultrasound frame.
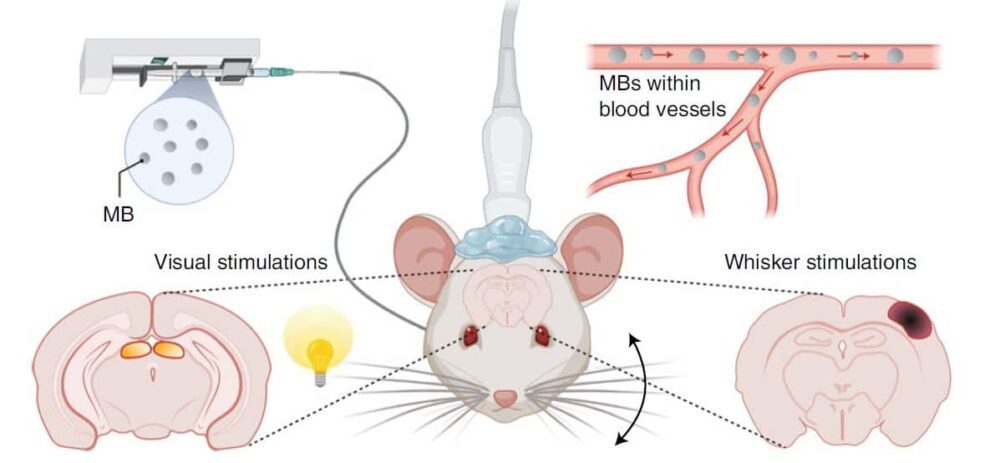
During ULM processing, the researchers saved every track with each microbubble position and its respective time position. They constructed ULM images by selecting a pixel size and sorting each microbubble within each pixel. Only pixels with at least five different microbubble detections during the total acquisition time were used for analyses.
The technique allowed the researchers to map functional hyperaemia (increased blood in the vessels) in both cortical and subcortical areas with 6.5 µm resolution. They quantified the temporal haemodynamic responses during whisker stimulations for four rats and during visual stimulations for three rats, by measuring the microbubble flux and velocity.
The team quantified the involvement of blood vessels during functional hyperaemia. They observed increases in microbubble count, speed and diameter for a representative arteriole and venule (very small arteries/veins leading into/out of the capillaries), noting that control animals did not exhibit any changes. They also introduced a “perfusion” and “drainage area index” to quantify further the involvement of each individual blood vessel. These increased by 28% and 54% during stimulation for the arteriole and venule, respectively.
Due to the large field-of-view, the researchers could perform quantitative analyses simultaneously for every vessel across the whole rat brain slice image, even in deep structures such as the thalamus for whisker stimulations and superior colliculus for visual stimulations.
“The achieved spatiotemporal resolution enables fULM to image different vascular compartments in the whole brain and to discriminate their respective contributions, in particular in the precapillary arterioles known to have a major contribution to vascular changes during neuronal activities,” write the authors.
Ultrafast ultrasound maps tiny blood vessels deep in the human brain
They add: “fULM shows that the relative increase in microbubble flow is greater in intra-parenchymal vessels rather than in arterioles. fULM also confirms depth-dependent characteristics for blood flow and speed in penetrating arterioles at baseline, and highlights a depth-dependent variation in blood speed during activation. It also quantifies large increases of microbubble flux, blood speed and diameter in venules during activation.”
As a new imaging research tool, fULM provides a way to track dynamic changes during brain activation and will offer insights into neural brain circuits. It will aid the study of functional connectivity, layer-specific cortical activity and or neurovascular coupling alterations on a brain-wide scale.
Tanter notes that researchers at Institute Physics for Medicine are collaborating with the Paris-based medical technology company Iconeus, to make this technology available for the neuroscience community and for clinical imaging very rapidly.












