Neurodegenerative diseases, characterized by a progressive loss of brain function, result primarily from synaptic loss and neuronal cell death in the central nervous system. Alzheimer’s disease (AD) is arguably the most common neurodegenerative disorder leading to dementia. The etiology and pathogenesis of AD are incompletely understood, and effective, disease-modifying drug treatments are lacking.
Prior work has shown that genetic, environmental, and age-related factors, along with alterations in energy metabolism, autophagy, and synaptic function, all contribute to the pathogenesis of AD. A new study by scientists at سکریپس ریسرچ اور میساچوسٹس انسٹی ٹیوٹ آف ٹیکنالوجی کے (MIT) has found a clue to the molecular cause of الزائمر. This clue may also explain why women are at greater risk for the disease.
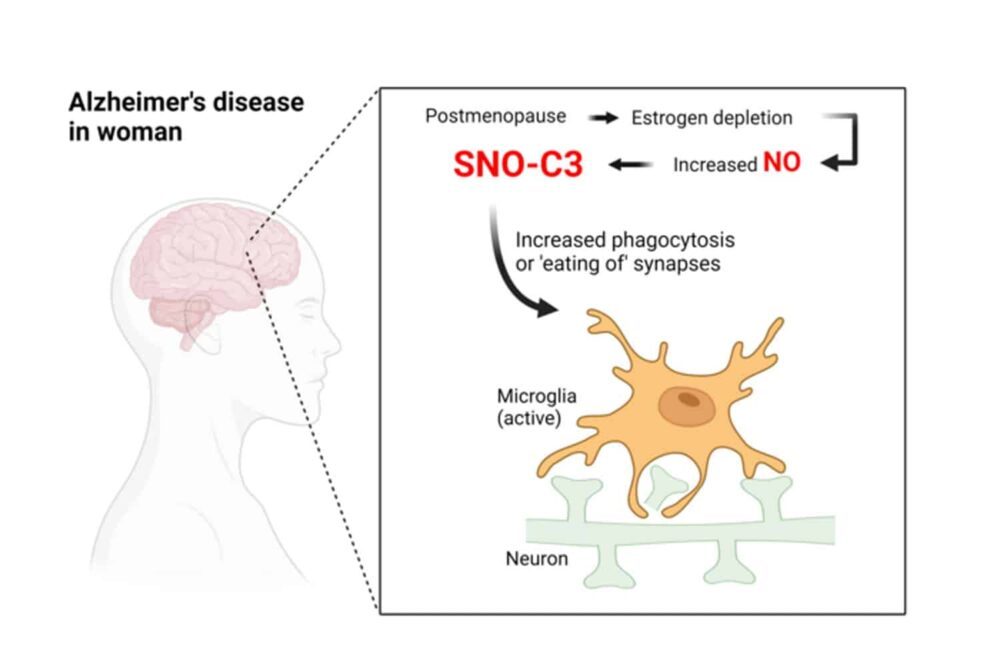
Scientists discovered that the brains of women who had died from the condition had considerably greater concentrations of complement C3, a hazardous inflammatory response protein that has undergone chemical modification. Additionally, they demonstrated that estrogen typically protects against forming this type of complement C3.
Study senior author Stuart Lipton, MD, Ph.D., professor and Step Family Foundation Endowed Chair in the Department of Molecular Medicine at Scripps Research said, "ہمارے نئے نتائج سے پتہ چلتا ہے کہ تکمیلی نظام کے ایک جزو کی کیمیائی ترمیم الزائمر کو بڑھانے میں مدد کرتی ہے، اور یہ وضاحت کر سکتی ہے، کم از کم جزوی طور پر، یہ بیماری بنیادی طور پر خواتین کو کیوں متاثر کرتی ہے۔"
لپٹن کی لیبارٹری بائیو کیمیکل اور سالماتی عمل کی تحقیقات کرتی ہے، جیسے کہ پروٹین ایس نائٹروسیلیشن، جس کے نتیجے میں تکمیلی C3 کا ایک ترمیم شدہ ورژن ہوتا ہے، جو اس کی وجہ ہو سکتا ہے۔ neurodegenerative عوارض. This chemical process, which produces a modified “SNO-protein” when a nitric oxide (NO)-related molecule attaches securely to a sulphur atom (S) on a specific amino acid building block of proteins, was first found by Lipton and his coworkers. Small clusters of atoms, like NO, frequently modify proteins in cells.
These modifications typically activate or inactivate a target protein’s functions. Because of technical difficulties, S-nitrosylation has received less attention than other protein modifications. Still, Lipton hypothesises that the “SNO-storms” of these proteins may play a significant role in the development of Alzheimer’s disease and other اعصابی بیماریوں.
In the latest study, the scientists measured the number of proteins changed in 40 postmortem human دماغ using brand-new techniques for identifying S-nitrosylation. The brains were divided equally between males and females, with half coming from persons who had died of Alzheimer’s disease and the other half coming from people who hadn’t.
The scientists found 1,449 distinct proteins that had been S-nitrosylated in these brains. Numerous proteins that have already been connected to Alzheimer’s disease were among those that were most often altered, including complement C3. Surprisingly, female Alzheimer’s brains had S-nitrosylated C3 (SNO-C3) levels that were more than six times higher than those of male Alzheimer’s brains.
The complement system is an older component of the human immune system in terms of evolution. The “complement cascade” comprises a family of proteins, including C3, that can activate one another to cause inflammation. For more than 30 years, scientists have known that, when compared to neurologically healthy brains, Alzheimer’s brains exhibit higher amounts of complement proteins and other inflammatory indicators.
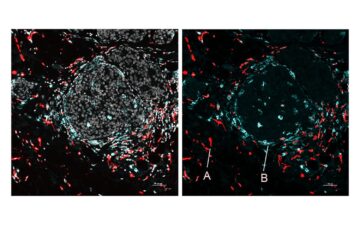
More recent studies have demonstrated, in particular, how complement proteins can cause brain-resident immune cells known as microglia to degrade synapses. At these junctions, نیورسن communicate with one another. Loss of synapses is a substantial correlate of cognitive decline in Alzheimer’s disease, and many scientists now assume that this synapse-destroying mechanism at least partially drives the disease process.
Why would female brains with Alzheimer’s have a higher prevalence of SNO-C3? The scientists proposed the hypothesis that estrogen explicitly protects women’s brains from C3 S-nitrosylation—and that this protection is lost when oestrogen levels sharply decline with menopause. There has long been evidence that the female hormone estrogen can have brain-protective effects under some circumstances.
This theory was validated by experiments using cultured human brain cells, which showed that SNO-C3 increases as estrogen (-estradiol) levels fall due to activating an enzyme that produces NO in brain cells. This rise in SNO-C3 triggers the degradation of synapses by microglia.
Study senior author Stuart Lipton, MD, Ph.D., نے کہا, "خواتین کو الزائمر ہونے کا زیادہ امکان کیوں ہوتا ہے یہ ایک طویل عرصے سے ایک معمہ رہا ہے، لیکن میرے خیال میں ہمارے نتائج اس پہیلی کے ایک اہم حصے کی نمائندگی کرتے ہیں جو میکانکی طور پر خواتین کی عمر کے ساتھ ساتھ ان کے بڑھتے ہوئے خطرے کی وضاحت کرتا ہے۔"
“Mechanistic insight into female predominance in Alzheimer’s disease based on aberrant protein S-nitrosylation of C3.”
جرنل حوالہ:
- Hongmei Yang et al. Mechanistic insight into female predominance in Alzheimer’s disease based on aberrant protein S-nitrosylation of C3. سائنس ایڈوانسز. ڈی او آئی: 10.1126/sciadv.ade0764